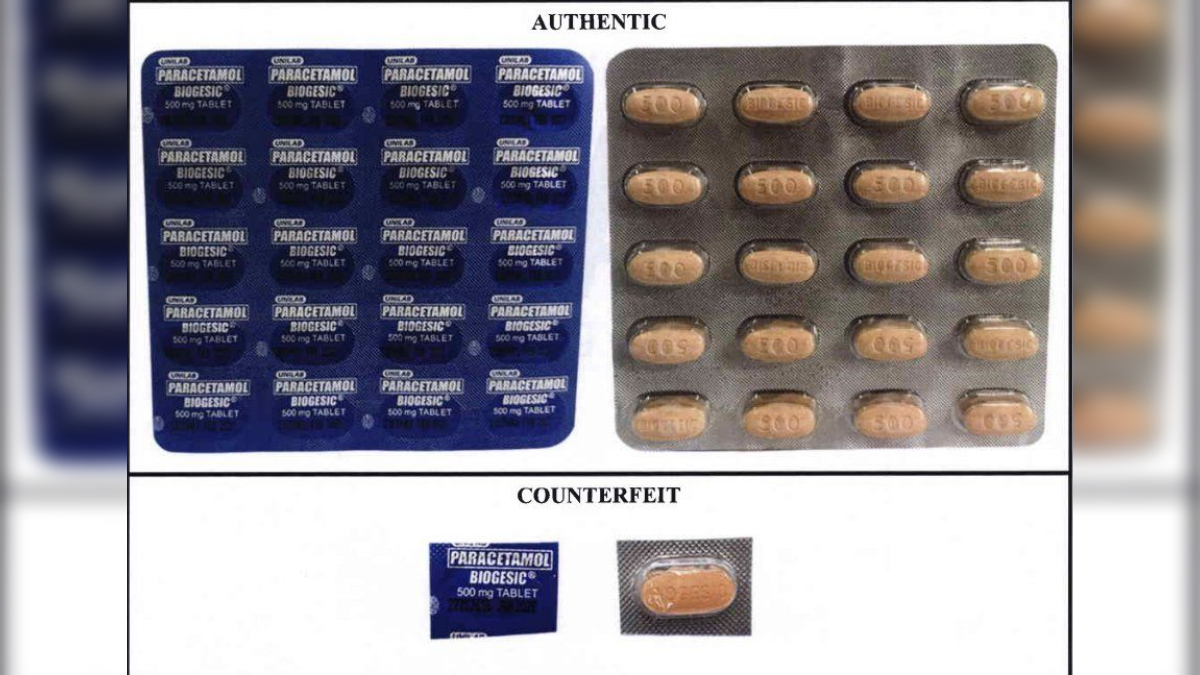
The Food and Drug Administration (FDA) has issued a public health warning about counterfeit versions of the popular paracetamol brand Biogesic being sold in the market. The advisory, released on July 4, highlights the potential dangers these fake medicines pose to consumers.
According to the FDA, the counterfeit Biogesic products can be distinguished by differences in lot numbers, capsules, packaging ridges (knurling), and print appearance when compared to the registered product. The agency urges consumers to purchase medicines only from FDA-licensed establishments to ensure safety.
The FDA has called on local governments and law enforcement agencies to take action against the sale of these counterfeit products. Penalties will be imposed on those found selling them. Reports of unregistered medicines or health products can be made to the FDA’s Center for Drug Regulation and Research at (02) 8809-5596 or via email at ereport@fda.gov.ph.
United Laboratories (Unilab), the manufacturer of Biogesic, also encourages consumers to report suspected counterfeit products by providing the lot/batch number, expiry date, purchase details, and clear photos of the items.
The FDA previously linked the spread of fake medicines to social media and online shopping platforms, which have facilitated their sale.