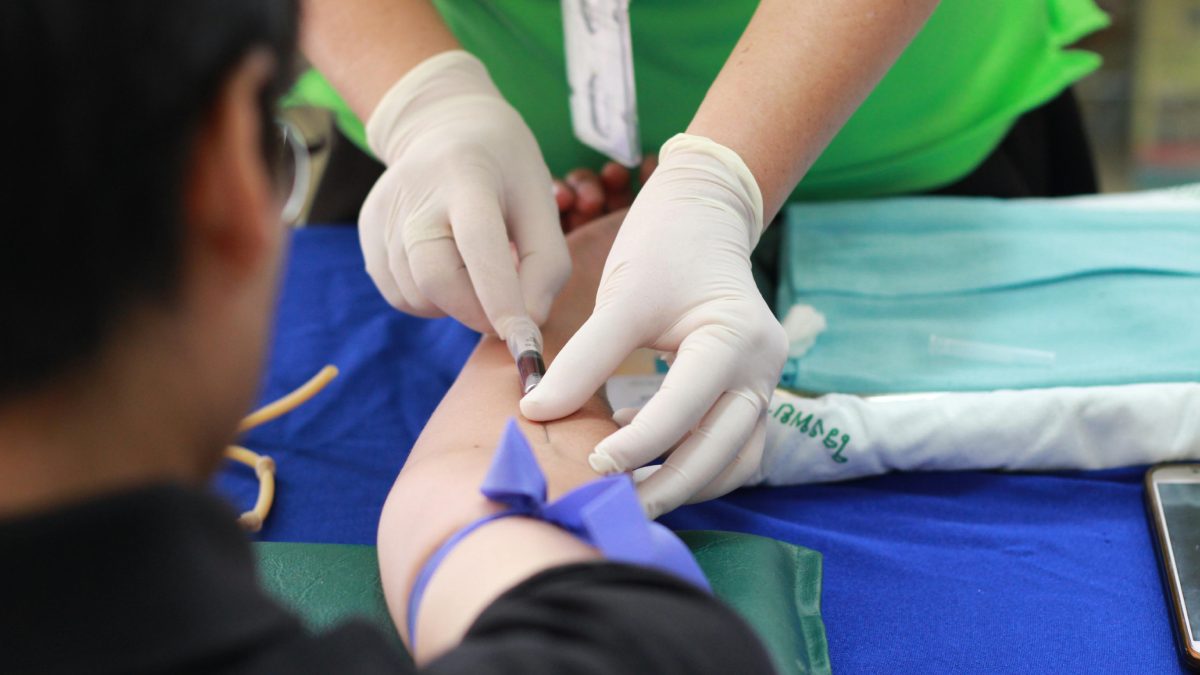
A groundbreaking small-scale trial of an experimental vaccine targeting aggressive triple-negative breast cancer has delivered encouraging results, as reported in Genome Medicine. The study involved 18 newly diagnosed patients who had not experienced metastasis. Each participant received three doses of a personalized vaccine after undergoing chemotherapy and tumor-removal surgery.
Three years following the treatment, 16 out of the 18 patients remained cancer-free. Although the trial did not have a control group, researchers highlighted that typically, only about 50% of patients receiving standard care would remain cancer-free over the same period. The vaccine, tailored to the specific genetic mutations in each patient’s tumor, targeted neoantigens—new proteins formed due to tumor DNA mutations.
Dr. William Gillanders of Washington University School of Medicine in St. Louis, who led the trial, expressed optimism. “The results were better than we expected,” he noted in a Reuters report, emphasizing that they are continuing with randomized controlled trials to directly compare outcomes with and without the vaccine.
An experimental technology has shown promise in addressing the challenges of replacing large defective genes in muscular dystrophy, researchers shared in Science. The innovative method, tested in mice, allows for the delivery of two separate halves of a gene, which are rejoined within the cells using RNA molecules called ribozymes. This “StitchR” technology enables cells to recognize and reassemble the split genes, producing functional proteins.
In muscular dystrophy cases such as Duchenne, current gene therapies often use truncated versions of genes due to size limitations. The StitchR method restored essential muscle proteins, such as Dystrophin and Dysferlin, in mice models, suggesting potential future applications for types of muscular dystrophy that currently lack effective treatments. Douglas Anderson of the University of Rochester School of Medicine, who led the study, said, “We are working towards treatments for some of the most debilitating genetic diseases on the planet.”
New clinical trial findings suggest that preserving the vagus nerve during surgery for early-stage stomach cancer can significantly improve post-surgical quality of life. The trial, which included 264 patients undergoing lower third stomach removal, found that nerve-sparing surgeries drastically reduced post-operative complications.
One year post-surgery, only 0.8% of the nerve-preserved group experienced gastroparesis—compared to 7.6% in the non-preserved group. Additionally, gallstones, a common complication, were noted in 6.8% of the usual surgery group but were absent in the nerve-preservation group.
While the preservation technique required more time, the study, published in JAMA Surgery, confirmed there were no increased risks of complications or cancer progression.